Projects
Single cell analyses to resolve heterogeneous CTC populations.
Circulating tumor cells are highly heterogeneous, containing a mixture of viable metastasis precursors, cells with either proliferative markers or markers of drug resistance and plasticity, as well as cells at various stages of anoikis or apoptosis. Single cell genomic and epigenetic studies are allowing us to dissect these populations and better understand the ways in which cancers adapt to stress and become competent to metastasize through the bloodstream.
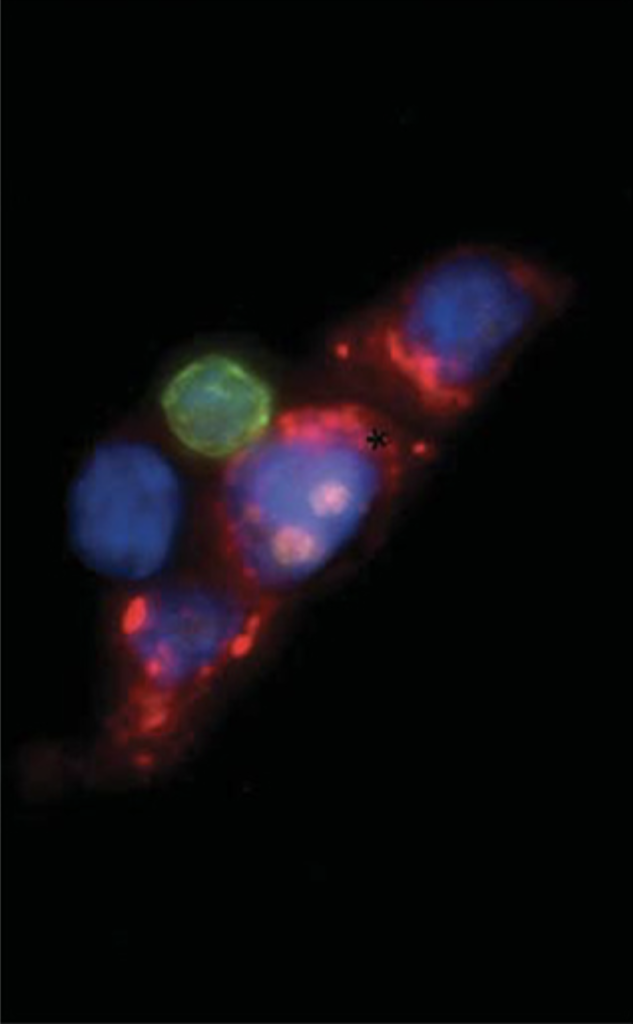
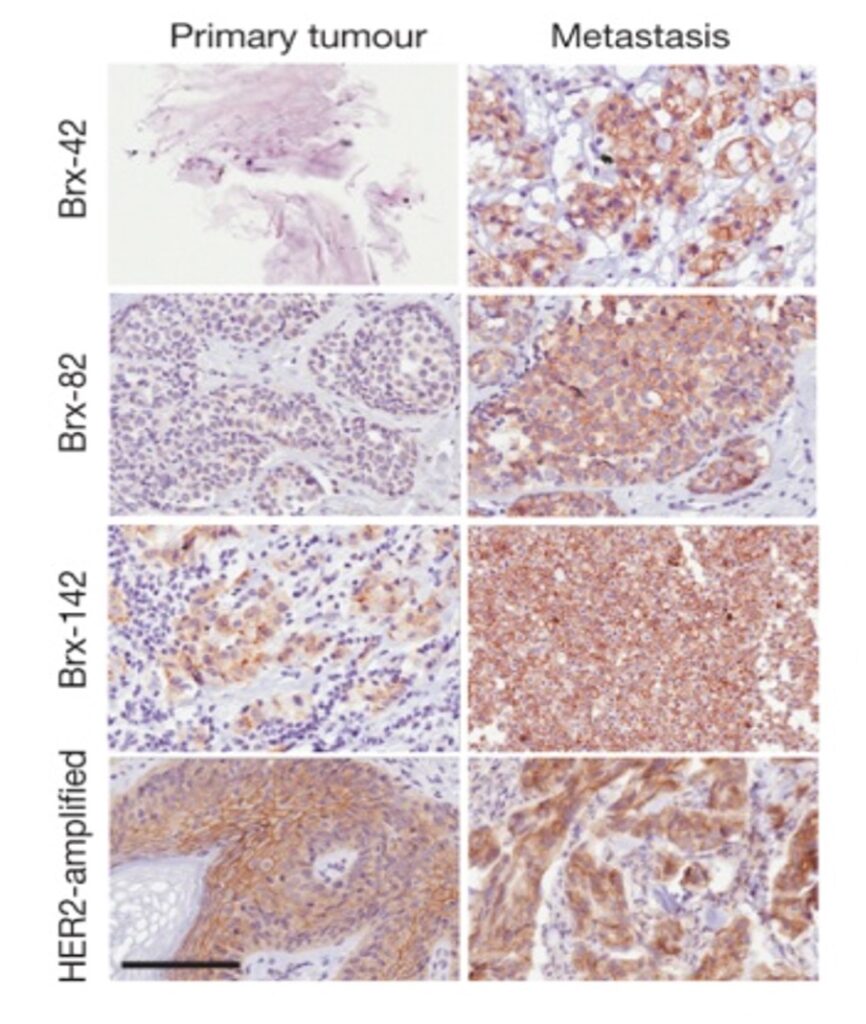
Novel therapeutic approaches for refractory breast cancer.
Metastatic breast cancer may be initially highly responsive to therapy, but following serial treatments, breast cancer cells acquire a high degree of resistance to multiple interventions. We have successfully cultured CTCs isolated from blood specimens of women with refractory breast cancer, and have established these in vitro as models of resistant disease. Cultured CTCs are highly tumorigenic, show a great degree of cell plasticity, but they also display unique vulnerabilities reflecting their distinct cell states. We are exploring cultured CTCs as models of refractory breast cancer, using both genetic and chemical screens for therapeutic interventions.
How do CTCs overcome ROS stress in the bloodstream?
The bloodstream has high oxygen content, compared with hypoxic tumor tissues, presenting CTCs with additional ROS stress as they circulate to distant sites. Understanding how cancer cells overcome ROS and other stresses in the bloodstream is of major interest, and it may offer opportunities to suppress blood-borne cancer metastasis.
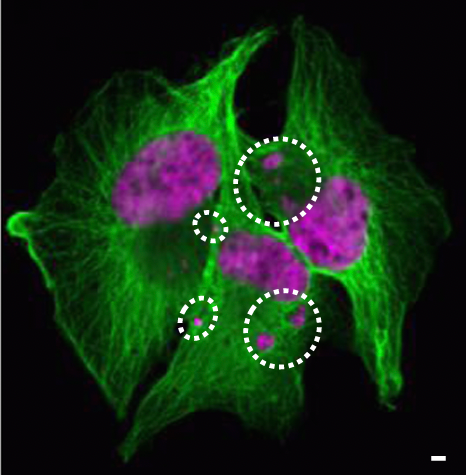
Understanding tumor cell dormancy and senescence.
In many cancers, including breast cancer, CTCs may lodge in distal tissues as Disseminated Tumor Cells, or “DTCs”, where they may remain quiescent for years, before initiating metastatic growth. The mechanisms that drive this dormancy and the ultimate reactivation of cancer growth are unknown. Using cultured CTC models, we have recapitulated such cell states and are exploring key regulatory pathways.
CTC analyses to monitor tumor evolution during cancer therapy.
Effective targeted therapies may shape the evolution of cancer cells, with initial tumor shrinkage followed by cancer cell adaptation, further epigenetic and genetic alterations and regrowth. Successful treatment of cancer thus requires “real time” monitoring for the development of heritable changes in tumor cells that may drive resistance to previous therapies, but also herald the acquisition of sensitivity to different treatments. Serial on-treatment biopsies of metastatic lesions have played an important role in rational cancer therapies, but they involve invasive procedures, and they are limited to sampling a single metastatic site. In contrast, liquid biopsies offer the potential for repeated and representative analysis of all tumor deposits. While DNA sequencing of ctDNA is preferred for rapid mutational analysis, CTC isolation and analyses enable measurements of RNA signaling to assess drug pharmacokinetics, cell surface protein expression to quantify expression of antibody and immune cell targets, as well as single cell resolution of emerging resistant clones. Ongoing studies, in partnership with clinical services at MGH, are designed to optimize CTC monitoring as part of first-in-human clinical trials.
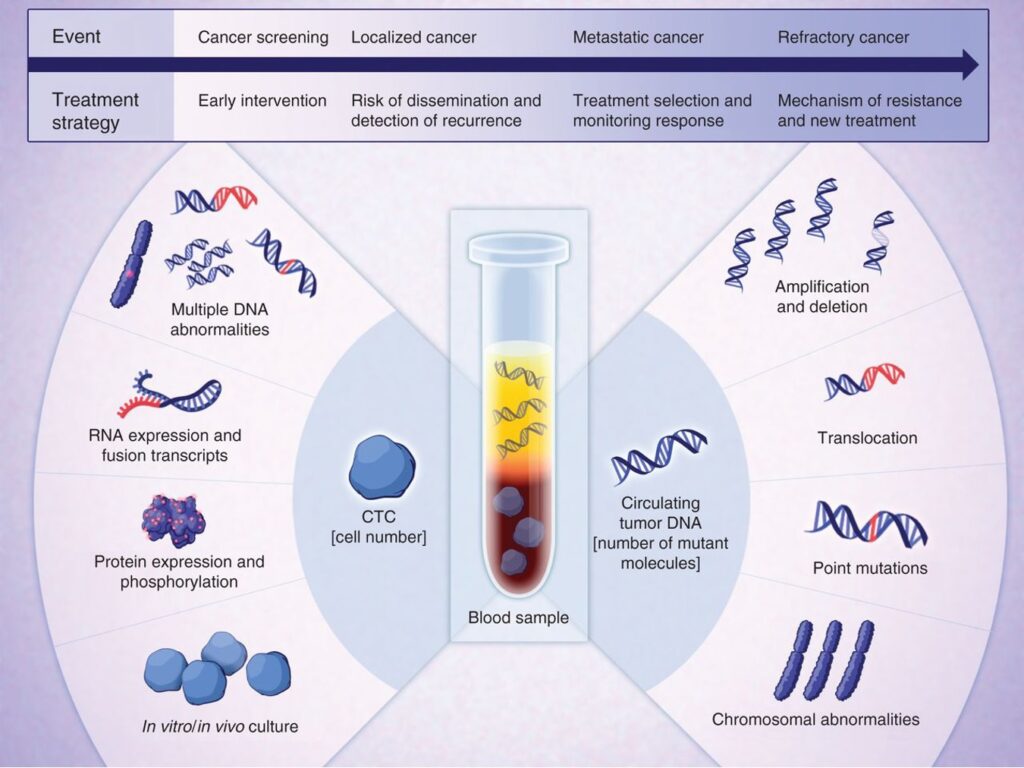
Blood-based early detection of invasive cancers.
Early blood-based detection of invasive cancer holds the promise of curative treatment for localized tumors. CTCs are shed into the vasculature, long before metastatic disease is established, and may serves as critical markers of invasive localized cancer. Current strategies for multi-cancer detection based on ctDNA methylation, fragment length variation and mutation content are promising, but suffer from insufficient accuracy for reliable identification of early cancers in healthy populations. A combination of multiple orthogonal assays will likely be required to achieve the level of sensitivity and specificity needed for preventing screening approaches. In partnership with the Toner lab at MGH, we are testing high blood volume CTC isolation and high sensitivity molecular markers, with the goal of providing the specificity and tissue-of-origin information critical to effective population cancer screening.